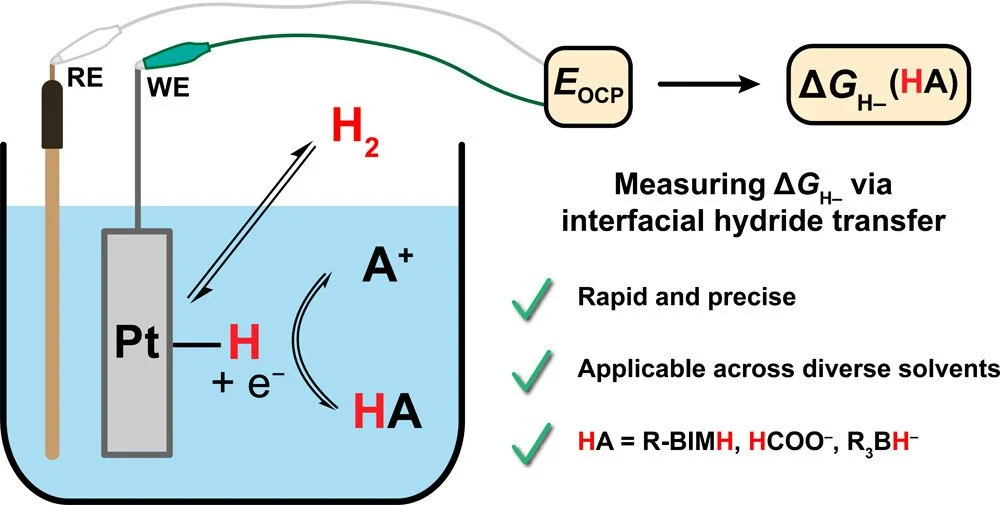
Reversible Interfacial Hydride Transfer as a Complementary Tool To Measure Molecular Hydricity
Hydride transfer is an essential elementary reaction across the chemical value chain, but there are limited methods available for quantifying thermodynamic hydricity (ΔG(H–)), particularly among main group reagents. Herein, we exploit facile H₂ activation and reversible hydride transfer from a metal surface to a molecular reagent, the net hydrogen reduction reaction (HRR), to develop a potentiometric method for quantifying ΔG(H–) of main group reagents recalcitrant to conventional methods. HRR potentiometry is first validated with a benzimidazole-based hydride donor and then applied to uncover the impact of the reaction environment on hydricity. For a benzimidazole-based hydride donor, HRR equilibrium potentials are roughly invariant across solvents, indicating that the solvent dependence of its hydricity largely reflects the differential solvation of H– across media. For formate, HRR potentials and corresponding hydricities depend strongly on water content. For borohydrides, HRR potentiometry reveals that effective hydricity values are strongly influenced by Lewis acid–base adduct formation with the hydride acceptor but are minimally influenced by the countercation. Together with these studies, the advantages, limitations, and practical considerations of the HRR potentiometry method are discussed, highlighting the power of this methodology as a complementary tool to measure molecular hydricity.
Chung, H. W.; Wang, H.-C.; Desai, S. P.; Müller, A. V.; Sena, S.; Glusac, K. D.; Concepcion, J. J.; Surendranath, Y. Reversible Interfacial Hydride Transfer as a Complementary Tool To Measure Molecular Hydricity, J. Am. Chem. Soc., 2025, 147 (40), 36291-36300. https://doi.org/10.1021/jacs.5c09582