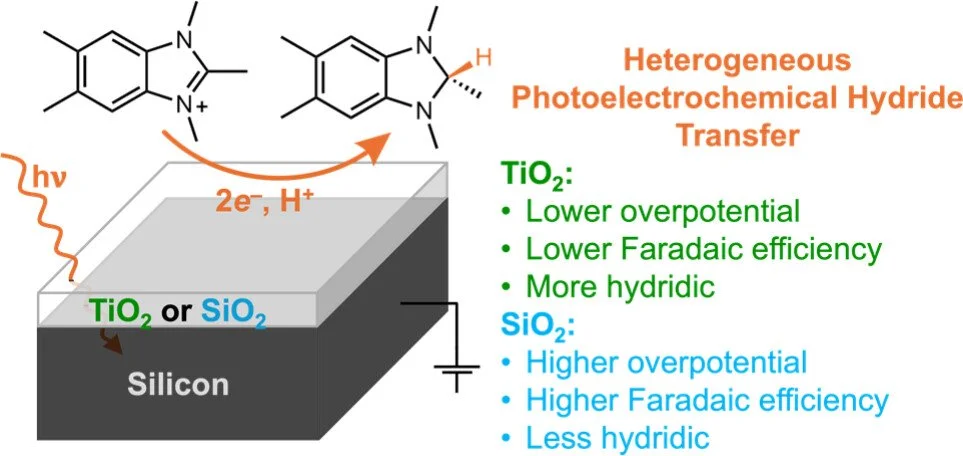
Photoelectrochemical Hydride Generation with Oxide-Coated Silicon
Photoelectrochemical generation of a potent organic hydride donor at silicon is demonstrated. Two different oxide-coated p-type silicon photoelectrodes reduced 1,2,3,5,6-pentamethyl-1H-benzo[d]imidazol-3-ium hexafluorophosphate, [PMBI][PF₆], to its corresponding imidazole hydride, PMBIH, in the presence of a proton source. Under 1 sun illumination, in acetonitrile with 1,5-diazabicyclo[4.3.0]non-5-ene (DBN) buffer, the p-Si photoelectrodes convert PMBI⁺ to PMBIH with good Faradaic efficiencies (FEs): 78% FE at −2.3 V vs Fc(+/0) for Si|TiO₂ and 83% FE at −2.6 V vs Fc(+/0) for Si|SiO₂ (where Si|SiO₂ represents silicon coated with an oxide layer). Generally, the Si|TiO₂ catalyzed the reaction at milder potentials than Si|SiO₂, but the Si|SiO₂ had better selectivity for PMBIH generation over H₂ evolution than Si|TiO₂. In light of prior studies of these photoelectrodes, the differences in selectivity and onset potential suggest a difference in mechanism, likely an outer-sphere electron transfer (ET) mechanism at the SiO₂ surface and potentially a proton-coupled ET process at the TiO₂ surface. To help understand reaction efficiency and identify areas of improvement, a thermochemical model for understanding net hydride transfer from the semiconductor to an acceptor in solution was developed. We find that the reactions in the present system are quite downhill. The high overpotentials (even while maintaining selectivity over H₂ evolution) emphasize the need for improved catalysts. This approach to evaluate the thermodynamics of net hydride transfer should be broadly valuable for electrochemical and photoelectrochemical processes.
Nedzbala, H. S.; Powers, R. E.; Knapp, A. S.; Yang, H.; Gentile, R.; Vecchi, P.; Dickenson, J. C.; Sirlin, J. T.; Donley, C. L.; Chua, K.; Griffin, P.; Müller, A. V.; Sampaio, R. N.; Jackson, M. N.; Parsons, G. N.; Concepcion, J. J.; Cahoon, J. F.; Meyer, G. J.; Miller, A. J. M.; Dempsey, J. L.; Mayer, J. M. Photoelectrochemical Hydride Generation with Oxide-Coated Silicon, J. Am. Chem Soc., 2025, 147 (41), 37123-37132. https://doi.org/10.1021/jacs.5c08666