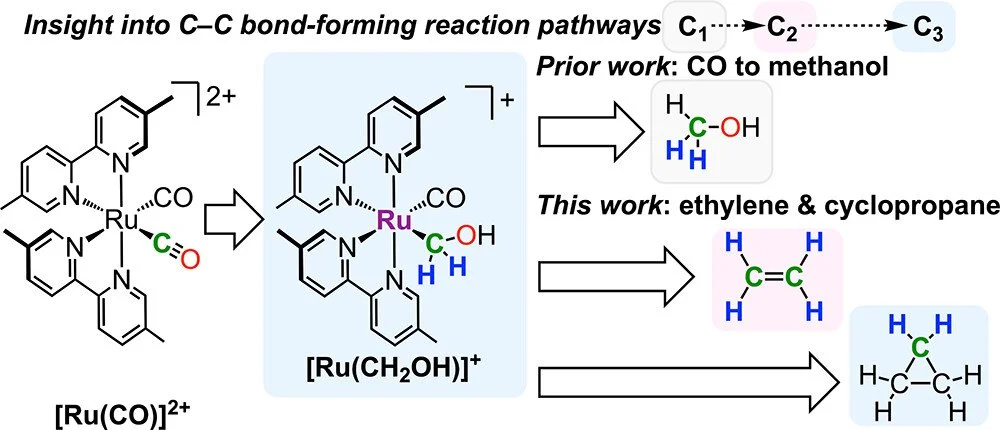
CO Reduction to Ethylene and Cyclopropane via a Trappable Ruthenium Methylidene
Ruthenium complexes based on cis-[Ru(bpy)₂(CO)₂]²⁺ (bpy is 2,2′-bipyridine) can reduce CO₂ and CO to C1 products including methanol, but access to products containing C–C bonds has been elusive. A reaction pathway to convert CO into multicarbon products ethylene and cyclopropane is presented here, along with mechanistic studies elucidating the key intermediates in C–C bond formation. The ruthenium hydroxymethyl complex [Ru(bpy′)₂(CO)(CH₂OH)]⁺ (bpy′ = 5,5′-dimethyl-2,2′-bipyridine) undergoes protonolysis to generate the ethylene complex [Ru(bpy′)₂(CO)(C₂H“₄”)]²⁺ even at −80 °C, with free ethylene released upon warming to room temperature. Experimental evidence implicates a highly electrophilic methylidene complex [Ru(bpy′)₂(CO)(CH₂)]²⁺ as the key intermediate. The methylidene was successfully trapped with nitriles and pyridine, forming adducts (ylide complexes) that each have a unique reactivity profile. With an appropriate nitrile, the adduct can be characterized at low temperature before warming generates ethylene. A more stable pyridine adduct [Ru(bpy′)₂(CO)(CH₂pyridine)]²⁺ was crystallographically characterized. Even ethylene itself is sufficiently nucleophilic to react with the electrophilic methylidene, revealing a route from CO to the C3 hydrocarbon cyclopropane. The methods for controlling the reactivity of hydroxymethyl and methylidene complexes toward C–C bond formation can inform the development of CO and CO₂ reduction catalysts.
Smith, A.; Tereniak, S.; Cox, H.; Massey, M.; Schauer, C.; Miller, A. CO Reduction to Ethylene and Cyclopropane via a Trappable Ruthenium Methylidene, J. Am. Chem. Soc., 2025, 147 (42), 38365-38375. https://doi.org/10.1021/jacs.5c11327