Molecular Modifications of Crystalline Poly(triazine imide) for Advancing Its Structure–Property Relationships in Light-Driven Catalysis
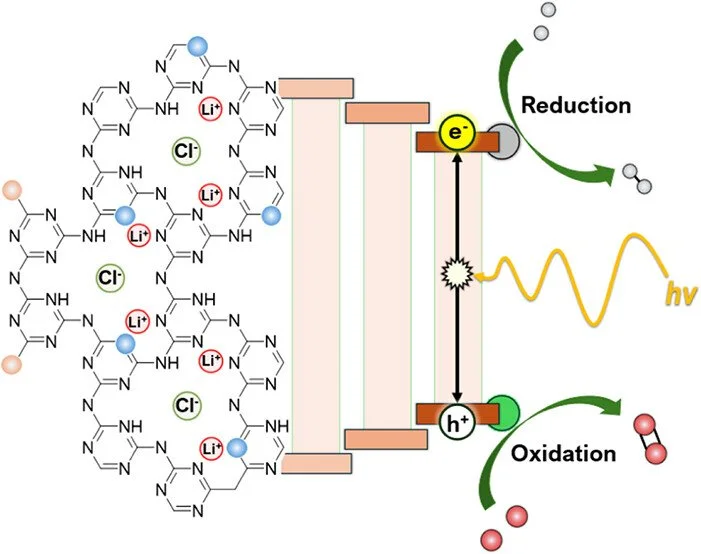
Carbon-nitride materials represent light-absorbing structures composed of earth-abundant elements capable of being leveraged for semiconductor photocatalysis at their surfaces. This study systematically investigates the addition of molecular modifiers to the synthesis of crystalline carbon nitrides to assess their effects on the materials’ structure, optical bandgap, and photocatalytic activity for hydrogen (H₂) and oxygen (O₂) evolution under ultraviolet and visible-light irradiation. Melamine and five pyrimidine-centered analogs were employed as building blocks to modify various heteroatoms within the polymeric framework. The modified materials were characterized with attention to the differences introduced by the monomeric modifiers and their influence on the resulting structures and compositions. The findings indicate that these changes significantly broaden the visible-light absorption range, albeit with the gradual loss of the bulk crystalline structure. As the loading of modifiers increased beyond 50%, a predominantly amorphous form of carbon nitride emerged. XPS, 13-C solid-state NMR, and SEM analyses corroborated the changes, which were attributed to modifications of the elemental composition and a reduced amount of Li cations and charge-balancing Cl anions owing to fewer binding sites in the intralayer cavities. In photocatalytic measurements under an ultraviolet 390 nm LED, and aided by photodeposited nanoparticle cocatalysts, the unmodified PTI-LiCl framework demonstrated the highest H₂ evolution rate (HER; 3.44 mmol·g⁻¹·h⁻¹) with an apparent quantum yield of 5.4%, along with total water splitting at rates of 163 μmol of H₂·g⁻¹·h⁻¹ and 75.6 μmol·O₂ g⁻¹·h⁻¹. While PTI-LiCl showed trace activity under a visible-light 440 nm LED, all modified materials exhibited enhanced reactivity with as low as 5% molecular modifiers. The photocatalytic rates peaked at a 15% modification level when using 2,4,6-triaminopyrimidine, with rates of 33 μmol·g⁻¹·h⁻¹for HER, along with 19.7 μmol of H2·g⁻¹·h⁻¹ and 8.7 μmol of O₂·g⁻¹·h⁻¹ for total water splitting. Density functional theory calculations were used to probe electronic structure changes resulting from the modifications. Thus, these results elucidate the structural, optical, and electronic changes arising from the five selected molecular modifiers and their impact on the semiconductors’ photocatalytic properties.
McGuigan, S.; Donley, C. L.; Ortega Ortiz, E.; O’Donnell, S.; Pauly, M.; Hockaday, W. C.; Stach, E. A.; Maggard, P. A. Molecular Modifications of Crystalline Poly(triazine imide) for Advancing Its Structure–Property Relationships in Light-Driven Catalysis, Chem. Mater., 2025, 37 (19), 7656-7670. https://doi.org/10.1021/acs.chemmater.5c01007