Cobalt(II) Phthalocyanine Substituents Tune the Electrocatalytic CO₂ Conversion to Methanol
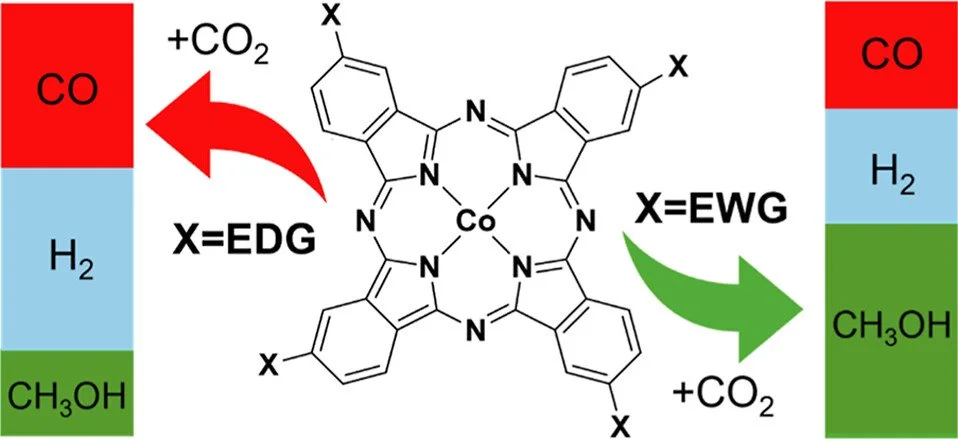
Cobalt phthalocyanine (Co(II)Pc) and its derivatives are promising molecular electrocatalysts for the electrochemical reduction of CO₂ to CO and methanol (CH₃OH). Despite increasing interest, a detailed mechanistic understanding of how ligand substituents influence catalytic activity, selectivity, and efficiency remains limited. In this study, we employ density functional theory (DFT) to systematically investigate the influence of electron-donating groups (EDGs) and electron-withdrawing groups (EWGs) on the electronic structure and redox properties of Co(II)Pc electrocatalyst, and to elucidate CO₂RR mechanistic pathways. Our results reveal that EWGs cause a positive shift in the reduction potentials, favor CO₂ binding over protonation of the Co metal center and promote downstream methanol formation at mild potentials. EDGs show opposite trends including favorable protonation steps, promoting a negative shift in the reduction potential, and facilitating the hydrogen evolution reaction (HER), competing with the desired CO₂RR pathway. Notably, CO dissociation is thermodynamically and kinetically unfavorable across all systems, positioning the redox potential versus CO dissociation energy as a key factor for methanol selectivity. These insights provide a predictive framework for rational catalyst design and underscore the critical role of electronic tuning in advancing molecular electrocatalysts for sustainable CO₂ conversion.
Barakat, M.; Fosu, E. A.; Jakubikova, E. Cobalt(II) Phthalocyanine Substituents Tune the Electrocatalytic CO2 Conversion to Methanol, Inorg. Chem., 2025, 64 (42), 20896-20906. https://doi.org/10.1021/acs.inorgchem.5c02279.