The importance of CO supersaturation and surface area—not geometry—for tandem single-catalyst CO₂ reduction to CH₃OH
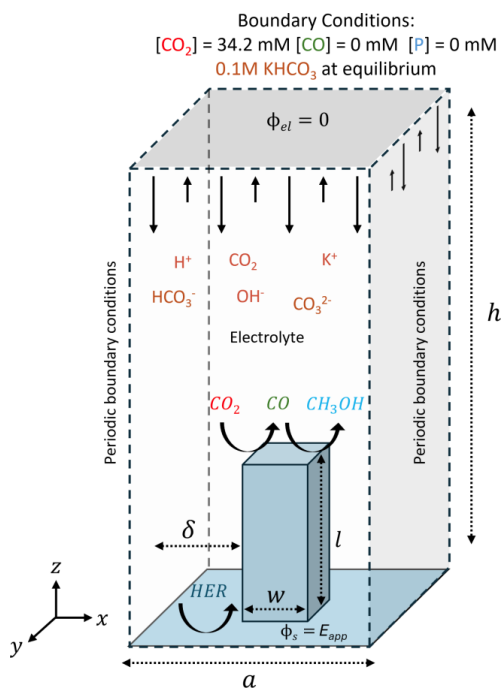
Effective electrochemical CO2 reduction to liquid fuels requires that the local catalytic environment facilitates the desired reactivity, yet a microscopic understanding of this environment is difficult to achieve from experiment alone. In this work, a 3D reaction-diffusion model was developed to explore the effects of electrode surface area and local geometry on the performance of a heterogeneous catalyst that performs a two-step CO₂ reduction cascade reaction to CO and then methanol under aqueous conditions. Kinetic parameters for the model were directly motivated by experimental results that used carbon electrodes functionalized with cobalt phthalocyanine (CoPc) catalysts. 3D architectures composed of arrays of square pillars with varying dimensions and either smooth or periodically modulated surfaces were tested, revealing the extent to which geometry modulates the performance of the cascade reactions. Surprisingly, the model illustrates that electrochemically-active surface area, regardless of electrode geometry, is the primary factor dictating the overall cascade reaction yield. Moreover, the results suggest that supersaturation of CO, with concentrations up to ten-fold higher than the equilibrium solubility limit, is critical for more efficient conversion to methanol. For any given geometry, the spatially averaged ratio of [CO] to [CO₂] is dictated by the electrochemically active surface area and determines the yield of methanol. For a fixed surface area, geometries that spatially confine the electrolyte yield moderate local [CO] to [CO₂] ratios within small volumes. In contrast, less confining geometries result in a broader distribution of local ratios spread over larger volumes with both configurations yielding the same spatially averaged [CO] to [CO₂] ratio. These insights provide valuable design principles—highlighting the critical importance of surface area and CO supersaturation—for engineering advanced electrode architectures that leverage intermediate trapping and CO supersaturation to enhance overall performance in tandem CO₂ reduction systems.
Fernandez, P.; Garcia-Batlle, M.; Shang, B.; Wang, H.; Parsons, G. N.; Cahoon, J. F.; Lopez, R. The importance of CO supersaturation and surface area—not geometry—for tandem single-catalyst CO₂ reduction to CH₃OH, 2025, ChemRxiv. https://doi.org/10.26434/chemrxiv-2025-743st