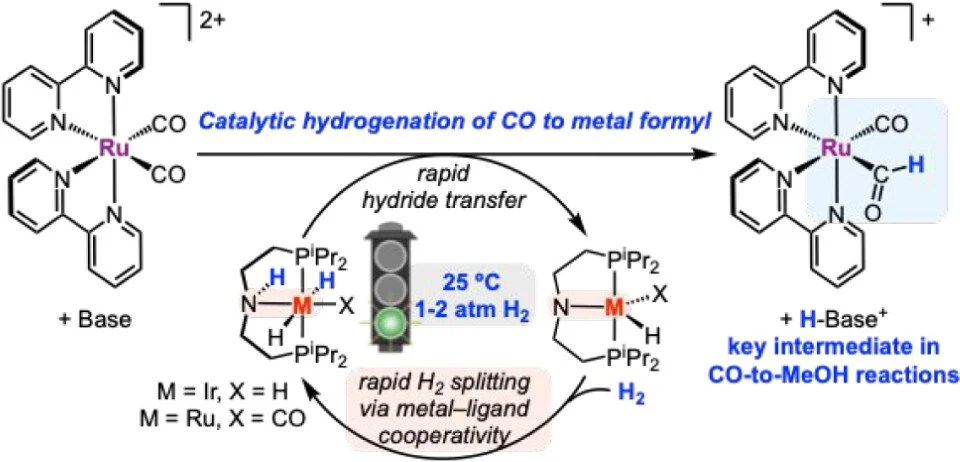
Catalytic Hydrogenation of a Ruthenium Carbonyl to Formyl Enabled by Metal–Ligand Cooperation
Metal formyl complexes are critical intermediates in the reduction of CO to valuable products such as methanol and higher alcohols/hydrocarbons, yet examples of formyl generation via the catalytic hydrogenation of transition metal carbonyl complexes under mild conditions are lacking. The catalytic hydrogenation of a ruthenium carbonyl complex with H₂ to produce a formyl complex is reported here. Two classes of hydrogenation catalysts were compared: bis(diphosphine)-ligated complexes that proceed via termolecular H₂ splitting with an external base and pincer-ligated complexes that proceed via an H₂ splitting mechanism involving metal–ligand cooperativity. The hydride transfer and H₂ splitting steps were evaluated for both classes of catalysts, revealing advantages for catalysts that utilize metal–ligand cooperativity and elucidating conditions to promote formyl generation. Only the pincer-ligated Ir and Ru complexes capable of reacting via pathways involving metal–ligand cooperativity were suitable for catalysis. Using 1–10 mol % of the catalysts (PNP)Ir(H)₂ and (HPNP)Ru(H)₂(CO) (PNP = (iPr₂PC2H4)₂N⁻), which use metal–ligand cooperation to activate H₂, up to 10 turnovers or up to 71% yield were achieved for the conversion of [Ru(bpy)₂(CO)₂]²⁺ (bpy = 2,2′-bipyridine) to the formyl complex [Ru(bpy)₂(CO)(CHO)]⁺. The Lewis acid B(C₆F₅)₃ was required as an additive to achieve high yields of the formyl complex using (HPNP)Ru(H)₂(CO) as a catalyst. The catalytic route avoids the use of expensive stoichiometric reagents, such as borohydride, instead generating metal formyls that are key intermediates in CO reduction schemes with H₂ gas.
Smith, A. M.; Fernández, S.; Tereniak, S. J.; Ahmad, S.; Kumar, A.; Chen, C.-H.; Hazari, N.; Ertem, M. Z.; Miller, A. J. M. Catalytic Hydrogenation of a Ruthenium Carbonyl to Formyl Enabled by Metal–Ligand Cooperation, ACS Catal., 2025, 15 (15), 13526–13533. https://doi.org/10.1021/acscatal.5c03137