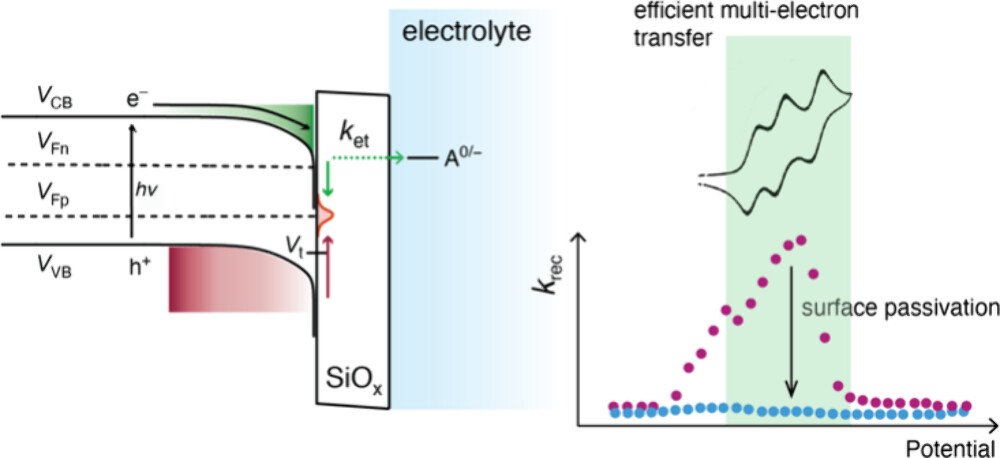
In Situ Characterization of Surface Recombination in p-Si/SiOₓ Based Photoelectrochemical Cells
Time-resolved infrared (TRIR) and electrochemical impedance spectroscopy (EIS) were utilized to quantify surface states present at silicon oxide (SiOₓ) protected crystalline p-Si electrolyte interfaces. The primary goal was to identify p-Si/SiOₓ photoelectrodes with both low surface recombination rates and efficient multi-electron transfer to an acceptor present in the external electrolyte. Three SiOₓ layers were investigated: native oxide (nOx), chemical oxide (cOx), and rapid thermal annealed (RTA) thermal oxide (tOx). Comparative study with [Ru(bpy)₃](PF₆)₂ as the electron acceptor indicated that tOx was most optimal with a small effective recombination rate, multi-electron transfer capability, and photovoltage of 500 ± 50 mV. A secondary goal was to analyze the surface recombination rates with the Shockley–Read–Hall (SRH) kinetic model. Two surface states were identified from this analysis, one closer to the CB edge (Vₜ,₁) and the other near the midgap (Vₜ,₂). EIS and SRH analyses revealed that a forming gas (5% H₂/N₂) anneal (FGA) decreased surface recombination for tOx and nOx through a lower density of surface states. In the case of tOx, the infrared data indicated that Vₜ,₂ was completely removed. The energetic positions of the band edges were correlated with the surface state density; low densities corresponded to more favorable potentials for inversion layer formation, which is expected to be most optimal for photocatalysis. Collectively this study indicates that the free carrier dynamics provided by TRIR represent a powerful in situ probe of the band edge and the surface state energetics in silicon based photoelectrochemical cells.
Vecchi, P.; Dickenson, J. C.; Gentile, R. J.; Powers, R. E.; Dempsey, J. L.; Grills, D. C.; Cahoon, J. F.; Sampaio, R. N.; Meyer, G. J In-situ Characterization of Surface Recombination in p-Si/SiOx Based Photoelectrochemical Cells. ACS Electrochemistry, 2025, 1 (8), 1500-1514. https://doi.org/10.1021/acselectrochem.5c00098