Substituents effects on the electrocatalytic CO₂ reduction by cobalt corroles in solution
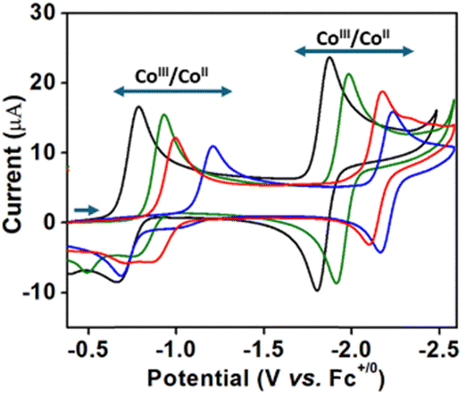
Electrochemical CO₂ reduction catalysis with cobalt corrole complexes in solution is reported. Corroles have attracted attention as contracted and trianionic tetrapyrrolic macrocycles that can be compared to leading porphyrin catalysts for CO₂ reduction, but most studies focus on heterogenized systems with poorly defined electrochemical responses. Electrochemical studies of cobalt corroles bearing axial triphenylphosphine ligands to ensure solubility are reported. The voltammetry provides mechanistic insights supporting CO₂ activation after the formal Co(II)/Co(I) reduction. The series of cobalt complexes, including a newly designed corrole with mixed perfluorophenyl/ortho-dimethoxyphenyl substituent pattern, provide evidence for electron-rich catalysts having stronger interactions with CO₂. The primary product of CO₂ reduction is CO, formed at a rate of ca. 90 s⁻¹.
Kumar, S.; Fernández, S.; Saltsman, I.; Fridman, N.; Mahammed, A.; Miller, A. J. M.; Gross, Z. Substituents effects on the electrocatalytic CO₂ reduction by cobalt corroles in solution, Chem. Commun., 2025, 61, 12924-12927. https://doi.org/10.1039/D5CC02717A