Unexpected Hydricity of a Bis-Carbene Iridium Complex Provides Insight into Electronic Structure Impacts on Hydride Donor Ability
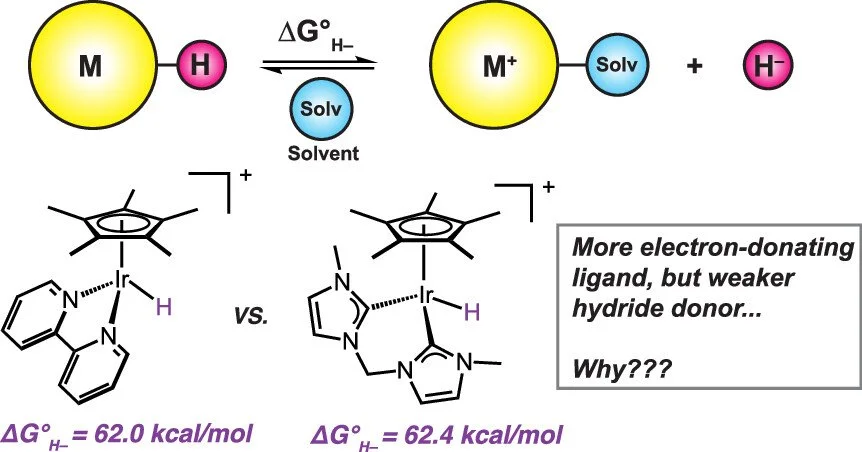
The hydricity (ΔG°(H–)), or hydride donor ability, of a transition metal complex is a thermodynamic parameter which can aid in the design and interpretation of various catalytic reactions that involve hydride transfer as a key step. In an attempt to generate a strong hydride donor, the bis-carbene ligand 3,3′-methylenebis(1-methyl-imidazol-2-ylidene) (“bis-mim”) was installed in an iridium hydride complex, [Cp*Ir(bis-mim)H]⁺. Experimental and computational studies show that [Cp*Ir(bis-mim)H]⁺ is actually a relatively weak hydride donor, however. To understand why the complex is an unexpectedly weak hydride donor, experimental and computational studies probing the steric and electronic effects on hydricity were conducted. Steric factors had a minimal impact on thermodynamic hydricity but dampened kinetic hydricity. The poor thermodynamic hydride donor ability can be attributed to an electronic structure that results in relatively long Cp*−Ir bonds, an unusually high Ir–H BDFE and pKₐ values that have an outsized influence on thermochemical cycles for hydricity.
Travis, B. D.; Ertem, M. Z.; Hearne, W. A.; Gonell, S.; Miller, A. J. M. Unexpected Hydricity of a Bis-Carbene Iridium Complex Provides Insight into Electronic Structure Impacts on Hydride Donor Ability, Inorg. Chem., 2025, 64 (34), 17255–17265. https://doi.org/10.1021/acs.inorgchem.5c02249