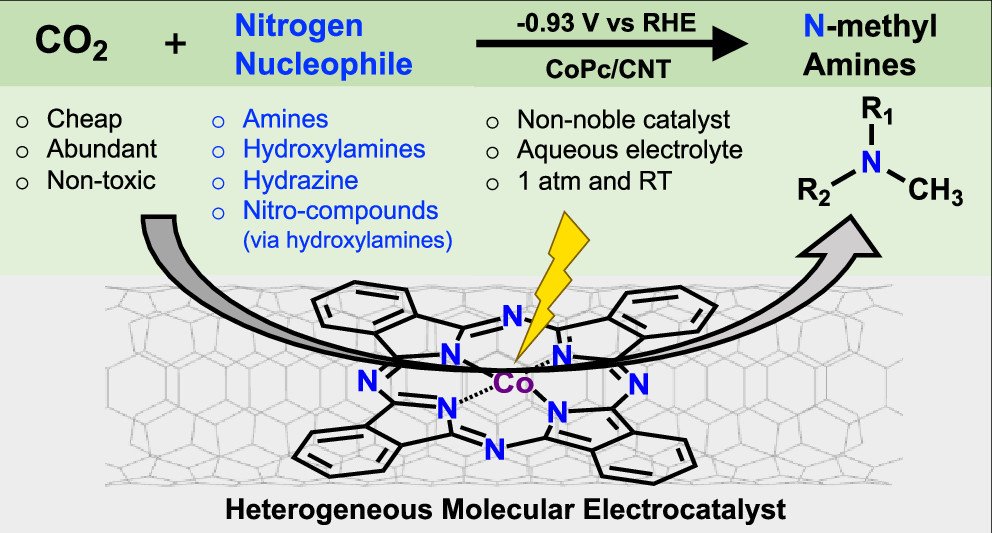
Electrochemical Reductive N-Methylation with CO Enabled by A Molecular Catalyst
The development of benign methylation reactions utilizing CO₂ as a one-carbon building block would enable a more sustainable chemical industry. Electrochemical CO₂ reduction has been extensively studied, but its application for reductive methylation reactions remains out of the scope of current electrocatalysis. Here we report the first electrochemical reductive N-methylation reaction with CO₂ and demonstrate its compatibility with amines, hydroxylamines, and hydrazine. Catalyzed by cobalt phthalocyanine molecules supported on carbon nanotubes, the N-methylation reaction proceeds in aqueous media via the chemical condensation of an electrophilic formaldehyde intermediate, formed from the four-electron reduction of CO₂, with nucleophilic nitrogenous reactants and subsequent reduction. By comparing various amines, we discover that the nucleophilicity of the amine reactant is a descriptor for the C-N coupling efficacy. We extend the scope of the reaction to be compatible with cheap and abundant nitro-compounds by developing a cascade reduction process in which CO2 and nitro-compounds are reduced concurrently to yield N-methyl amines via the overall transfer of 12 electrons and 12 protons.
Rooney, C.; Wu, Y.; Tao, Z.; Wang, H. Electrochemical Reductive N-Methylation with CO₂ Enabled by A Molecular Catalyst. J. Am. Chem. Soc. 2021, 143 (47), 19983-19991. https://doi.org/10.1021/jacs.1c10863