Electron Inversion and Tunneling at Silicon Thermal Oxide Interfaces for Solar-Driven Molecular Catalysis to Syngas
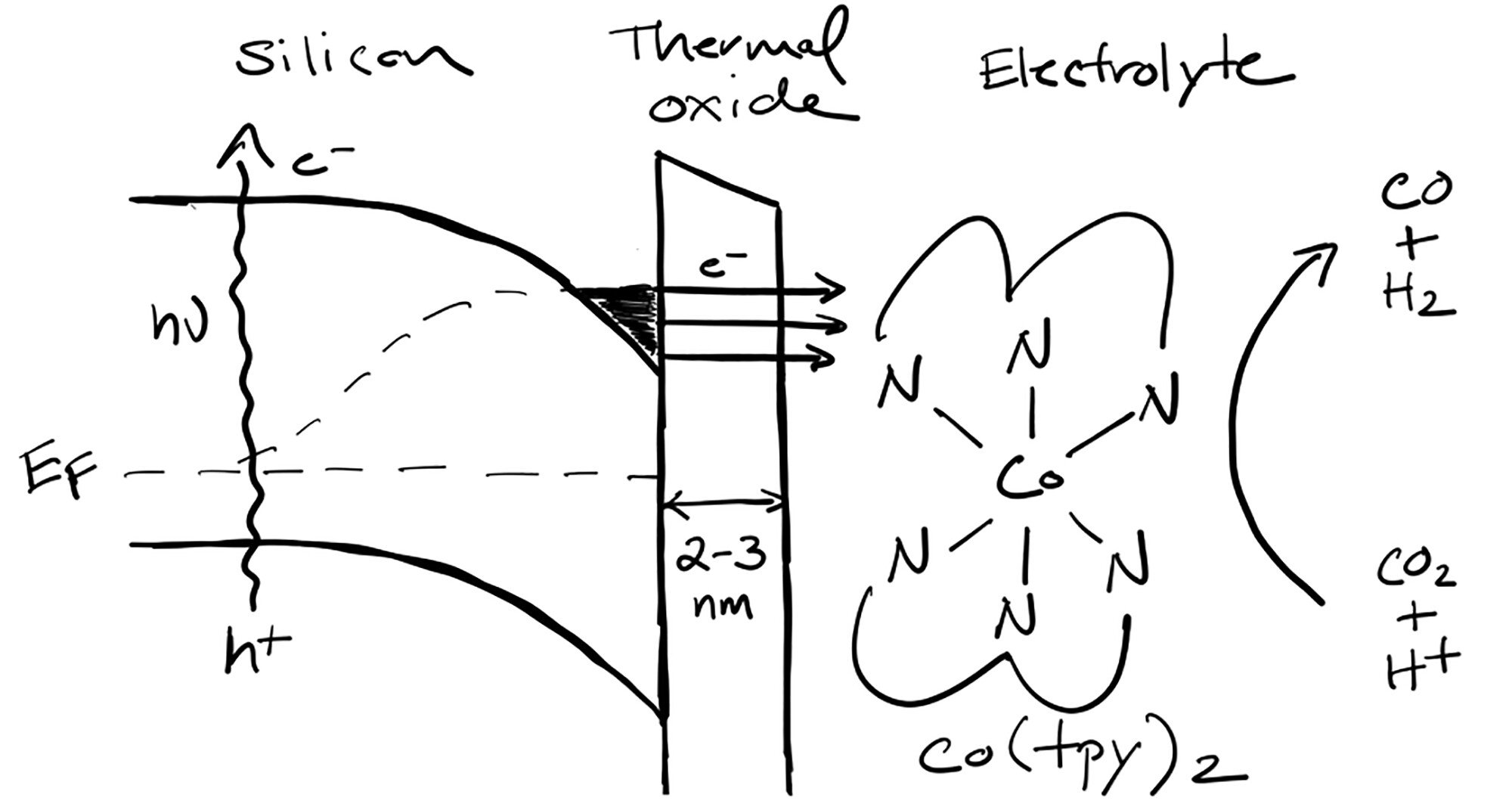
Semiconductor photoelectrodes are regularly coupled to solid-state heterogeneous catalysts to perform solar-driven reduction of CO₂. Less frequently, molecular catalysts are employed to better control the reactivity toward desired products, yet the development of robust semiconductor/molecule interfaces has proven challenging. Here, we demonstrate that a 2–3 nm thermal oxide layer on Si exhibits stability in aqueous solution, high photovoltage, and a photocurrent density of ∼10 mA/cm² for the solar-driven photoelectrochemical reduction of a homogeneous molecular catalyst, producing syngas with an ∼2:1 H₂ to CO ratio. Because of a low defect density, the oxide interface forms an electron inversion layer with metal-like electron density at cathodic potentials. This inversion layer facilitates electron transfer to redox-active molecules via tunneling even if the molecule’s reduction potential is beyond the semiconductor’s conduction band edge. Using an electrolyte solution composed of a homogeneous cobalt bis(terpyridine) catalyst in a water/organic solvent mixture, stable photoelectrochemistry was observed under 1-sun illumination, exhibiting an ∼30% Faradaic efficiency for CO that was similar to a glassy carbon electrode under comparable conditions. The results demonstrate that an ultrathin thermal oxide interface is a robust platform for development of aqueous-stable, molecule-driven photoelectrocatalysis.
He, S.; Bottum, S. R.; Dickenson, J. C.; Margavio, H. R. M.; Keller, N. D.; Oyetade, O. A.; Gentile, R. J.; Teitsworth, T. S.; Shin, S. J.; Dempsey, J. L.; Miller, A. J. M.; Sampaio, R. N.; Tereniak, S. J.; Donley, C. L.; Lockett, M. R.; Parsons, G. N.; Meyer, G. J.; Cahoon, J. F. Electron Inversion and Tunneling at Silicon Thermal Oxide Interfaces for Solar-Driven Molecular Catalysis to Syngas, J. Am. Chem. Soc., 2025, 147(13), 11145-11151. https://doi.org/10.1021/jacs.4c17251