Electrocatalytic Reductive Amination of Aldehydes and Ketones with Aqueous Nitrite
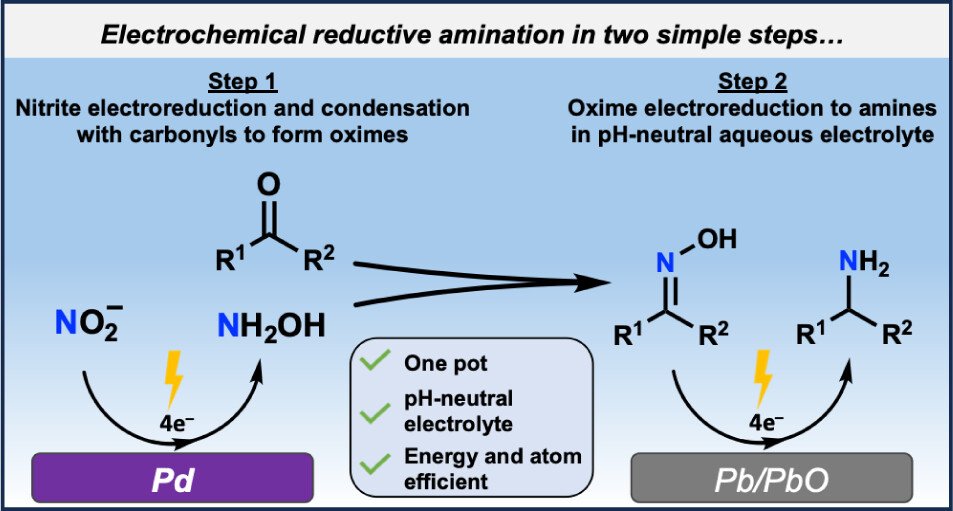
The electrocatalytic utilization of oxidized nitrogen waste for C–N coupling chemistry is an exciting research area with great potential to be adopted as a sustainable method for generation of organonitrogen molecules. The most widely used C–N coupling reaction is reductive amination. In this work, we develop an alternative electrochemical reductive amination reaction that can proceed in neutral aqueous electrolyte with nitrite as the nitrogenous reactant and via an oxime intermediate. We develop a selection criterion for nitrite reduction electrocatalysts suited for oxime electrosynthesis and, in doing so, find Pd to be a highly efficient catalyst for this reaction, reaching an oxime Faradaic efficiency of 82% at −0.21 V vs the reversible hydrogen electrode. The aliphatic or aromatic structure of the carbonyl reactant impacts the efficacy of the catalyst, with aromatic substrates leading to suppressed oxime formation and detrimental reduction of the carbonyl to the alcohol. We developed a Pb/PbO electrocatalyst that selectively performs oxime reduction in the neutral aqueous electrolyte. With acetone as a model substrate, we demonstrate an efficient one-pot, two-step electrochemical reaction for the conversion of acetone to isopropyl amine with 85% yield and 50% global Faradaic efficiency.
Rooney, C. L.; Sun, Q.; Shang, B.; Wang, H. Electrocatalytic Reductive Amination of Aldehydes and Ketones with Aqueous Nitrite, J. Am. Chem. Soc., 2025, 147(11), 9378-9385. https://doi.org/10.1021/jacs.4c16344