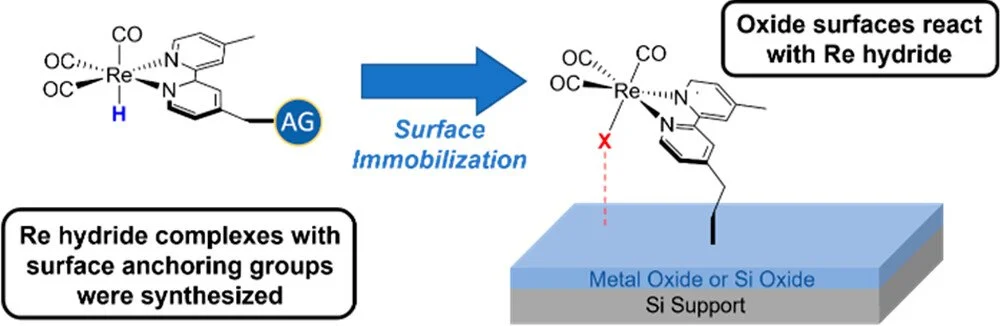
Synthesis and Surface Attachment of Molecular Re(I) Hydride Species with Silatrane Functionalized Bipyridyl Ligands
Three molecular Re hydrides of the form (ᴿbpy)Re(CO)₃H with 2,2′-bipyridine (bpy) ligands containing silatrane functional groups for surface attachment on metal oxide surfaces were synthesized. IR spectroscopy and cyclic voltammetry (CV) demonstrated that the complexes containing the silatrane functional groups have electronic properties similar to those of a control compound, which did not contain functional groups for attachment. Additionally, in a similar fashion to the control compound, the silatrane containing Re hydrides are electrocatalysts for the reduction of CO₂ to CO in solution. The silatrane containing complexes were immobilized on a thin layer of TiO₂ on Si, and the resulting composites were characterized using X-ray photoelectron and IR spectroscopy as well as cyclic voltammetry in the dark and under illumination. Control experiments indicated that the hydride complexes are not stable on the surface and degrade to species which contain a bpy ligand, three CO ligands, and an unknown ligand in the sixth site. Similarly, when one of the silatrane containing Re hydride complexes was immobilized on Si nanoparticles with a thin layer of SiO₂ or silica nanoparticles, the hydride ligand was lost. Density functional theory calculations were used to corroborate the observed behavior of hydride species on a surface. Overall, this work demonstrates the difficulties associated with attaching well-defined molecular hydride complexes to metal oxide surfaces.
Jia, X.; Cui, K.; Alverez-Hernandez, J. L.; Donley, C. L.; Gang, A.; Hammes-Sciffer, S.; Hazari, N.; Jeon, S.; Mayer, J. M.; Nedzbala, H. S.; Shang, B.; Stach, E. A.; Stewart-Jones, E.; Wang, H.; Williams, A. Synthesis and Surface Attachment of Molecular Re(I) Hydride Species with Silatrane Functionalized Bipyridyl Ligands. Organometallics, 2023, 42 (16), 2238-2250. https://doi.org/10.1021/acs.organomet.3c00235