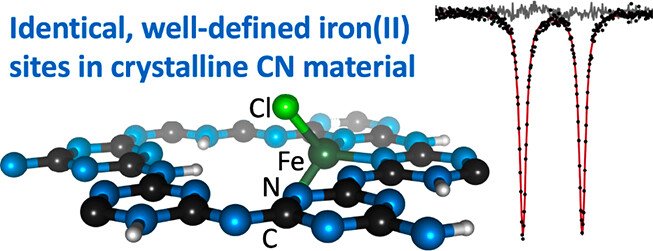
Well-Defined Iron Sites in Crystalline Carbon Nitride
Carbon nitride materials can be hosts for transition metal sites, but Mössbauer studies on iron complexes in carbon nitrides have always shown a mixture of environments and oxidation states. Here we describe the synthesis and characterization of a crystalline carbon nitride with stoichiometric iron sites that all have the same environment. The material (formula C₆N₉H₂Fe₀.₄Li₁.₂Cl, abbreviated PTI/FeCl₂) is derived from reacting poly(triazine imide)·LiCl (PTI/LiCl) with a low-melting FeCl₂/KCl flux, followed by anaerobic rinsing with methanol. X-ray diffraction, X-ray absorption and Mössbauer spectroscopies, and SQUID magnetometry indicate that there are tetrahedral high-spin iron(II) sites throughout the material, all having the same geometry. The material is active for electrocatalytic nitrate reduction to ammonia, with a production rate of ca. 0.1 mmol cm⁻² h⁻¹ and Faradaic efficiency of ca. 80% at −0.80 V vs RHE.
Genoux, A.; Pauly, M.; Rooney, C. L.; Choi, C.; Shang, B.; McGuigan, S. Fataftah, M. S.; Kayser, Y.; Suhr, S. C. B.; DeBeer, S.; Wang, H.; Maggard, P. A.; Holland, P. L. Well-Defined Iron Sites in Crystalline Carbon Nitride. J. Am. Chem. Soc. 2023, 145 (38), 20739–20744. https://doi.org/10.1021/jacs.3c05417.