Unusual Electrochemical Activity of Thin SiO₂ Layers Leads to Instability of Molecular Attachment in Hybrid Photoelectrodes
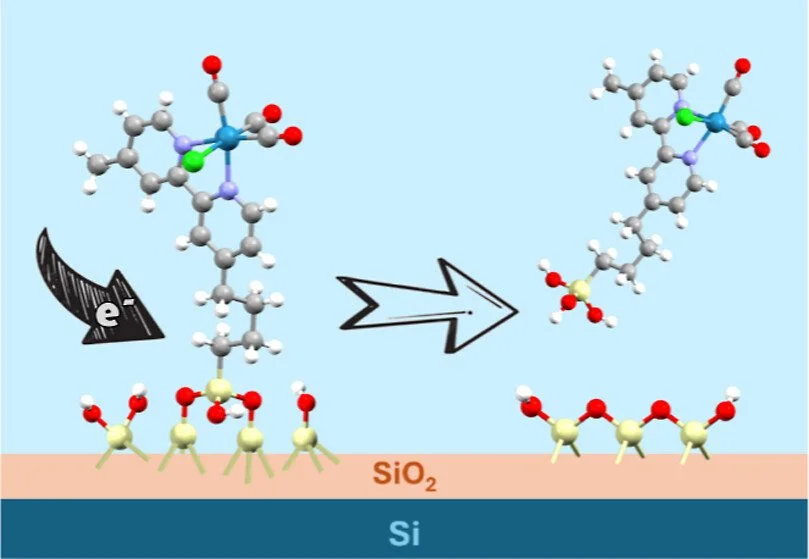
Hybrid photoelectrodes, comprised of a light-absorbing semiconductor and a surface-integrated molecular catalyst, are attractive for applications in artificial photosynthesis, since they combine the advantages of broadband semiconductor light absorption with the selectivity of molecular catalysis. A widely used class of hybrid photoelectrodes is based on Si substrates passivated by a thin (<3 nm) layer of silicon oxide, which is commonly prepared by controlled chemical or thermal oxidation, resulting in chemical oxide (ChO) or thermal oxide (ThO) layers, respectively. However, the electrochemical stability of these oxide layers, and the chemical stability of the semiconductor-molecule assembly in hybrid photoelectrodes, are not well understood, with evidence that covalently bound molecules detach from the oxide surface upon application of cathodic bias. We have examined the intrinsic electrochemical reactivity of silicon oxide layers and how it affects the attachment of molecular monolayers. We determined that the surface of Si|ThO is primarily terminated with hydrophobic siloxane moieties, whereas that of Si|ChO contains a higher concentration of hydrophilic silanol groups. Initial high current densities for Si|ChO under applied bias up to −2 V vs Ag/AgCl, decrease during repeated cyclic voltammetry scans, due to the consumption of surface-bound water. This is manifested by a reversible wave around −0.5 V in CH₃CN solution, and a similar pH-dependent wave in water, revealing the pKₐ of the silanol groups to be ∼4. Our combined observations support the electrochemically induced dehydration of the SiO₂ surface, which converts silanol groups to siloxanes and proceeds through an H atom intermediate that is most likely stabilized by pentavalent Si. We propose that similar reactivity is responsible for the electrochemical loss of alkylsiloxane-attached molecules under cathodic bias, which has important implications for the choice of catalyst attachment strategy in hybrid photoelectrodes.
Cappuccino, C.; Tanwar, M.; Jia, X.; Hazari, N.; Donley, C. L.; Fakhraai, Z.; Manbeck, G. F.; Grills, D. C.; Polyansky, D. E. Unusual Electrochemical Activity of Thin SiO2 Layers Leads to Instability of Molecular Attachment in Hybrid Photoelectrodes. ACS Appl. Energy Mater., 2025, In press. https://doi.org/10.1021/acsaem.5c03010