Reorganization Energies for Interfacial Proton-Coupled Electron Transfer to a Water Oxidation Catalyst
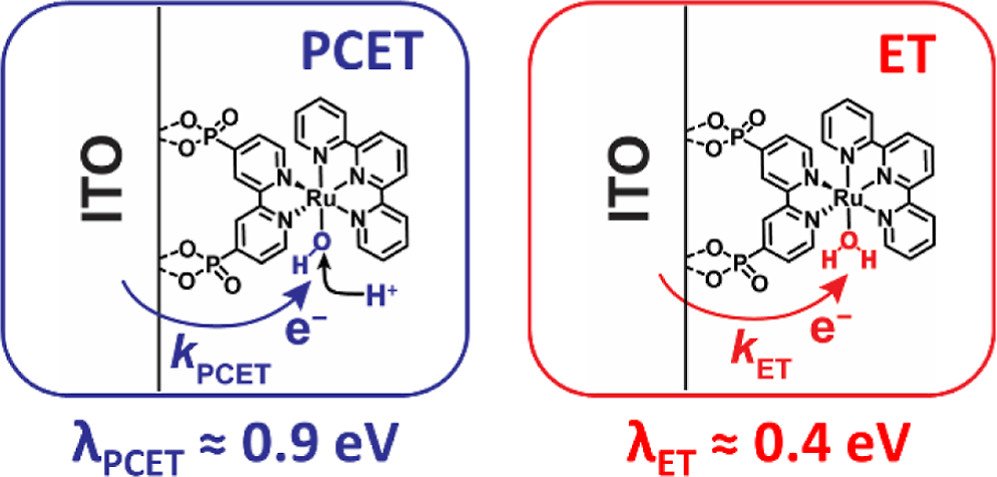
The reorganization energy (λ) for interfacial electron transfer (ET) and proton-coupled ET (PCET) from a conductive metal oxide (In₂O₃:Sn, ITO) to a surface-bound water oxidation catalyst was extracted from kinetic data measured as a function of the thermodynamic driving force. Visible light excitation resulted in rapid excited-state injection (kᵢₙⱼ > 108 s⁻¹) to the ITO, which photo-initiated the two interfacial reactions of interest. The rate constants for both reactions increased with the driving force, −ΔG°, to a saturating limit, kₘₐₓ, with rate constants consistently larger for ET than for PCET. Marcus–Gerischer analysis of the kinetic data provided the reorganization energy for interfacial PCET (0.90 ± 0.02 eV) and ET (0.40 ± 0.02 eV), respectively. The magnitude of kₘₐₓ for PCET was found to decrease with pH, behavior that was absent for ET. Both the decrease in kₘₐₓ and the larger reorganization energy for an unwanted competing PCET reaction from the ITO to the oxidized catalyst showcases a significant kinetic advantage for driving solar water oxidation at high pH. Computational analysis revealed a larger inner-sphere reorganization energy contribution for PCET than for ET arising from a more significant change in the Ru–O bond length for the PCET reaction. Extending the Marcus–Gerischer theory to PCET by including the excited electron–proton vibronic states and the proton donor–acceptor motion provided an apparent reorganization energy of 1.01 eV. This study demonstrates that the Marcus–Gerischer theory initially developed for ET can be reliably extended to PCET for quantifying and interpreting reorganization energies observed experimentally.
Kessinger, M.; Soudackov, A. V.; Schneider, J.; Bangle, R. E.; Hammes-Schiffer, S.; Meyer, G. J. Reorganization Energies for Interfacial Proton-Coupled Electron Transfer to a Water Oxidation Catalyst. J. Am. Chem. Soc. 2022, 144 (44), 20514–20524. https://doi.org/10.1021/jacs.2c09672