Photochemical Ligand-Based CO₂ Reduction Mediated by Ruthenium Formyl Species
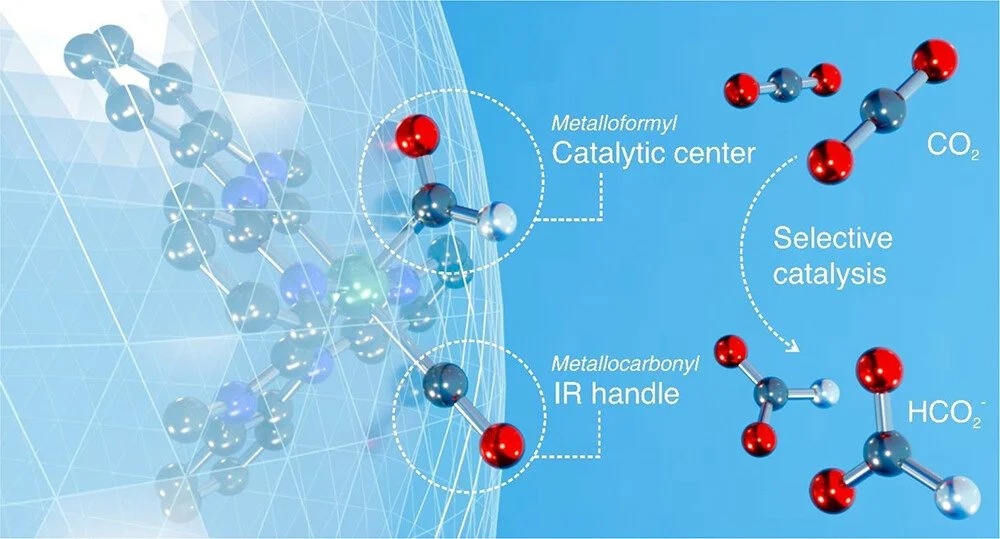
A new, ligand-based strategy for CO₂ reduction to formate has been demonstrated. This approach relies on the photochemical generation of a coordinatively saturated transient ruthenium metalloformyl species, Ru-CHO, capable of reducing CO₂ to free formate directly. Under this paradigm, a highly reactive radical cation which is capable of facile formal hydrogen atom transfer (HAT) is generated via reductive quenching of an excited-state photosensitizer. Sequential electron transfer (ET) and HAT steps to a ruthenium carbonyl complex subsequently yield the Ru-CHO species, which upon further reduction undergoes fast hydride transfer to CO₂, producing free formate. High formate selectivity (up to 98%) and impressive catalytic performance (TON ∼ 5300; TOF ∼ 0.1 s⁻¹) were observed. Detailed mechanistic studies revealed that the overall process is highly sensitive to the identity of the radical cation, with divergent reactivity observed when HAT thermodynamics are altered. These findings provide new insights into ligand-based hydride transfer mechanisms and establish a foundation for the rational design of selective CO₂ reduction catalysts.
Desai, S. P.; Müller, A. V.; Cappuccino, C.; Polyansky, D. E.; Grills, D. C.; Ertem, M. Z.; Concepcion, J. J. Photochemical Ligand-Based CO2 Reduction Mediated by Ruthenium Formyl Species, J. Am. Chem. Soc., 2025, 147(26), 22725–22733. https://doi.org/10.1021/jacs.5c04611