Electron Transfer Energetics in Photoelectrochemical CO₂ Reduction at Viologen Redox Polymer-Modified p-Si Electrodes
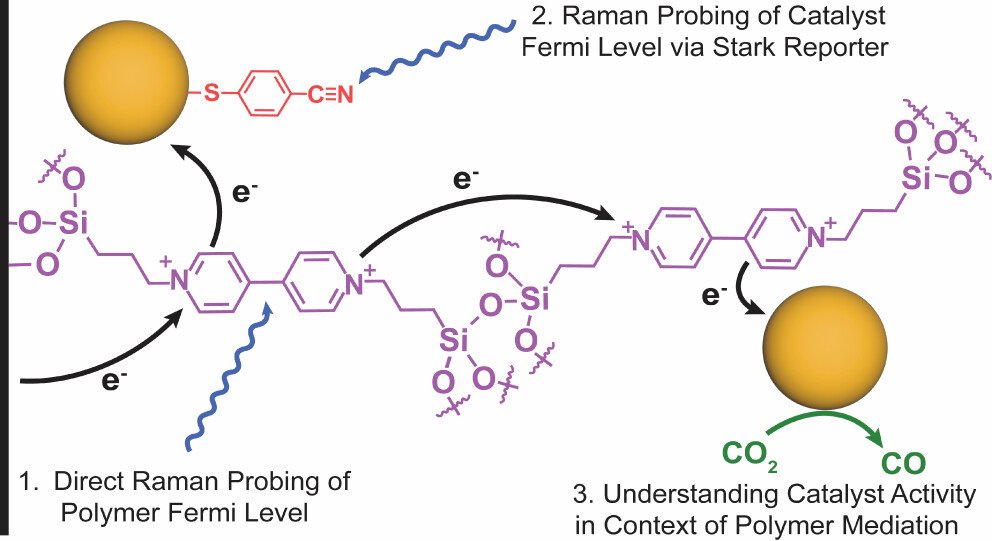
While redox polymer-mediated catalysis at silicon photoelectrodes has been studied since the 1980s, there have been few detailed studies of these materials in photoelectrochemical CO₂ reduction. Here, we develop silicon photoelectrodes functionalized with a viologen-based polymer that mediates the formation of catalytic gold nanoparticles. The presence of gold was confirmed by X-ray photoelectron spectroscopy (XPS), and the nanoparticles were imaged with high-angle annular dark field scanning transmission electron microscopy (HAADF-STEM). We probed the CO₂ reduction process during bulk photoelectrolysis to find modest, yet consistent CO faradaic efficiencies across a range of applied potentials. Operando surface-enhanced Raman spectroscopy (SERS) was used to measure the Fermi levels of both the viologen polymer and the Au catalyst sites. The operando measurement of the Fermi levels of all three components of the photocathode provides a unified picture of the electron transfer process in the semiconductor-redox polymer–catalyst system. The redox polymer serves as the electron transfer mediator between the Si substrate and Au sites. In addition, the Au Fermi level equilibrates with the Fermi level of the viologen polymer, which in turn fixes the quasi-Fermi level of Au catalysts at the p-Si/redox polymer interface. This suggests a potential future direction of using redox polymers with tunable potentials to modulate the potential of metal cocatalysts and thus control the reaction selectivity.
Sheehan, C. J.; Suo, S.; Jeon, S.; Zheng, Y.; Meng, J.; Zhao, F.; Yang, Z.; Xiao, L.; Venkatesan, S.; Metlay, A. M.; Donley, C. L.; Stach, E. A.; Lian, T.; Mallouk, T. E. Electron Transfer Energetics in Photoelectrochemical CO2 Reduction at Viologen Redox Polymer-Modified p-Si Electrodes, J. Am. Chem. Soc., 2025, 147(11), 9629-9639. https://doi.org/10.1021/jacs.4c17762