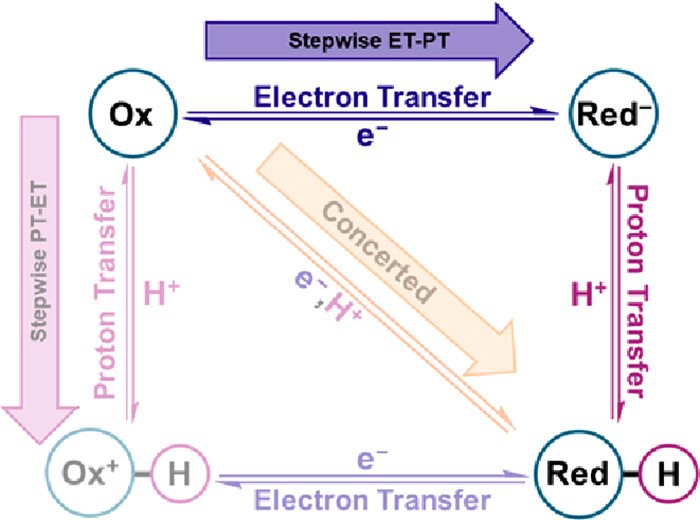
Direct Evidence for a Sequential Electron Transfer–Proton Transfer Mechanism in the PCET Reduction of a Metal Hydroxide Catalyst
The proton-coupled electron transfer (PCET) mechanism for the reaction Mox–OH + e
Kessinger, M. C.; Xu, J.; Cui, K.; Loague, Q.; Soudackov, A. V.; Hammes-Schiffer, S.; Meyer, G. J. Direct Evidence for a Sequential Electron Transfer–Proton Transfer Mechanism in the PCET Reduction of a Metal Hydroxide Catalyst, J. Am. Chem. Soc., 2024, 146 (3) 1742-1747. https://doi.org/10.1021/jacs.3c10742