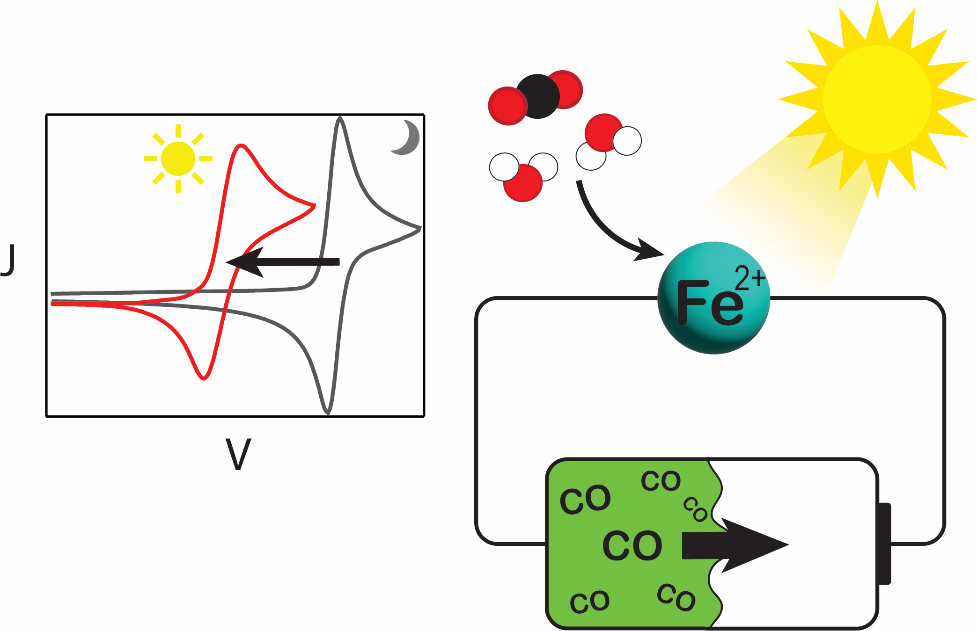
Photosynthesis of CO from CO₂ with an iron polypyridyl catalyst at a passivated silicon photoelectrode
A first-row transition metal catalyst, [Fe(tpy)(Mebim-py)(NCCH₃)]²⁺ (tpy = 2,2′:6′,2′′-terpyridine, Mebim-py = 1-methylbenzimidazol-2-ylidene-3-(2′-pyridine)) mediates CO₂ reduction to CO at passivated p-Si photoelectrodes with applied potentials 240 mV positive of the standard CO₂/CO reduction potential. The molecular catalyst's selectivity for CO was retained under photoelectrochemical conditions, with negligible direct proton reduction promoted by the photoelectrode. The faradaic efficiency for CO (44 ± 6%) was slightly enhanced relative to the catalyst performance in the dark (33%). A photosynthetic cell based on this photocathode system, coupled with ferrocene oxidation at the anode, successfully operated at a cell voltage of −1.2 V. The photovoltage generated by illumination of p-Si–CH₃ met and surpassed the potential required for CO₂ reduction when coupled with ferrocene oxidation. By leveraging a low-overpotential CO₂ reduction electrocatalyst, a photo-assisted electrochemical efficiency of 0.15% and applied bias photon-to-current efficiency of 0.05% was achieved for this single-junction cell, ultimately storing 46 kJ mol⁻¹ (11 kcal mol⁻¹) of photon energy.
Bein, G. P.; Fernandez, S.; Tereniak, S. J.; Sampaio, R. N.; Miller, A. J. M.; Dempsey, J. L. Photosynthesis of CO from CO₂ with an Iron Polypyridyl Catalyst at a Passivated Silicon Photoelectrode, Chem. Sci., 2025, Advance Article. https://doi.org/10.1039/D5SC05984D