Methyl Termination of p-Type Silicon Enables Selective Photoelectrochemical CO₂ Reduction by a Molecular Ruthenium Catalyst
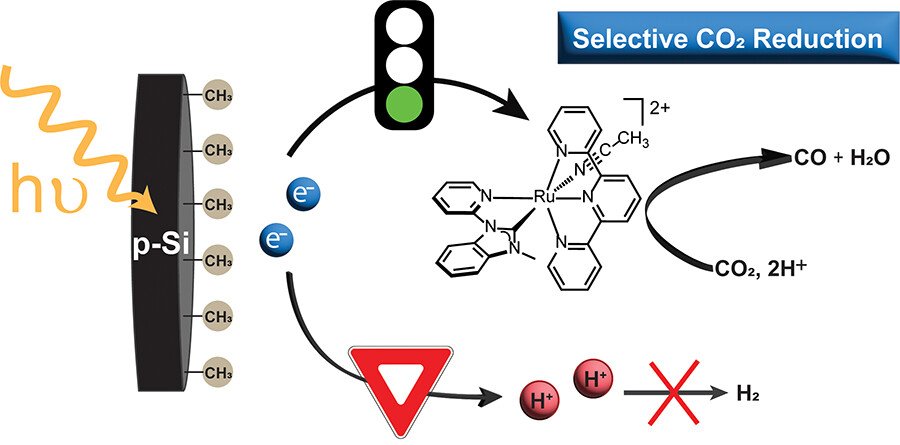
Methyl-terminated p-type silicon photoelectrodes selectively drive CO2 reduction by a homogeneous [Ru(tpy)(Mebim-py)(NCCH3)]2+ catalyst (tpy = 2,2′:6′,2″-terpyridine, Mebim-py = 1-methylbenzimidazol-2-ylidene-3-(2′-pyridine)). A 460 mV photovoltage is quantified for the photoelectrode. Under 1 sun illumination, this system achieves a Faradaic efficiency of 87% for CO at −1.7 V vs Fc+/0, matching reports of the same catalyst at metallic electrodes operating at −2.1 V. When 5% water is introduced, the CH3-terminated Si photoelectrode remains stable, selectivity for CO is retained, and current density increases. Methyl termination suppresses the competitive hydrogen evolution observed for H-terminated Si photoelectrodes, which under the same conditions produce ca. 60% CO and 8% H2 and have unstable performance. These results establish that a semiconductor photoelectrode can power a molecular CO2 reduction catalyst without hydrogen evolution by the photoelectrode itself. Methyl termination of p-Si allows CO2 reduction to kinetically outcompete proton reduction, revealing an important design principle for selective fuel formation.
Bein, G. P.; Stewart, M. A.; Assad, E. A.; Tereniak, S. J.; Sampaio, R. N.; Miller, A. J. M.; Dempsey, J. L. Methyl Termination of p-Type Silicon Enables Selective Photoelectrochemical CO₂ Reduction by a Molecular Ruthenium Catalyst. ACS Energy Lett., 2024, 9 (4), 1777-1785. https://doi.org/10.1021/acsenergylett.4c00122