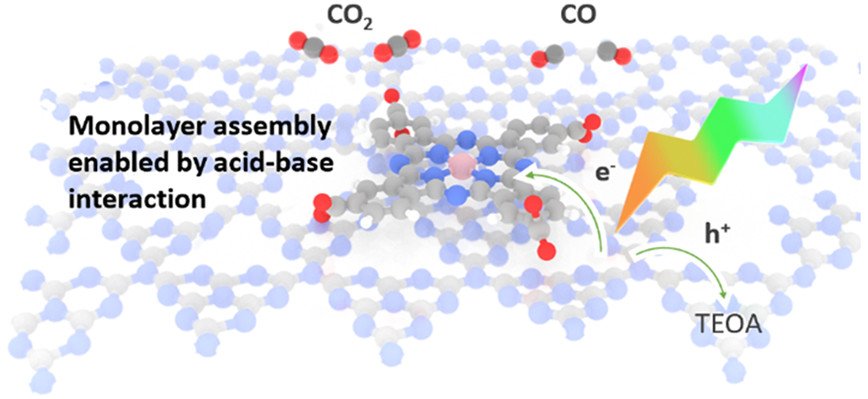
Monolayer Molecular Functionalization Enabled by Acid–Base Interaction for High-Performance Photochemical CO₂ Reduction
We report the development of a hybrid catalyst consisting of carbon nitride (CNₓ) and cobalt phthalocyanine tetracarboxylic acid (CoPc-COOH), which converts CO₂ to CO with high reaction rate (1067 μmol/g·h) and high selectivity (over 98%), under simulated solar irradiation. The carboxylic acid substituents on the phthalocyanine ligands play a critical role as they bind to the amine groups of CNₓ to enable nearly ideal monolayer coverage of the molecular cocatalyst on the semiconductor surface and promote catalytic activity from the molecular complex. Specifically, the CNₓ/CoPc-COOH hybrid material achieves a reaction rate 16 times higher than a CNₓ material containing unsubstituted CoPc molecules. We further show that activation and deactivation of the CNₓ/CoPc-COOH composite, which are associated with the reduction and decomposition of CoPc-COOH, respectively, both proceed at a nearly constant rate regardless of the CO₂ reduction reaction rate. The decoupling of charge carrier injection and CO₂ reduction catalysis has important mechanistic implications for future performance optimization and materials design of photocatalysts for CO₂ reduction.
Shang, B.; Zhao, F.; Choi, C.; Jia, X.; Pauly, M.; Wu, Y.; Tao, Z.; Zhong, Y.; Harmon, N.; Maggard, P. A.; Lian, T.; Hazari, N.; Wang, H. Monolayer Molecular Functionalization Enabled by Acid–Base Interaction for High-Performance Photochemical CO₂ Reduction. ACS Energy Letters 2022, 7, 2265-2272. https://doi.org/10.1021/acsenergylett.2c01147