Ethanol Upgrading to n-Butanol Using Transition-Metal-Incorporated Poly(triazine)imide Frameworks
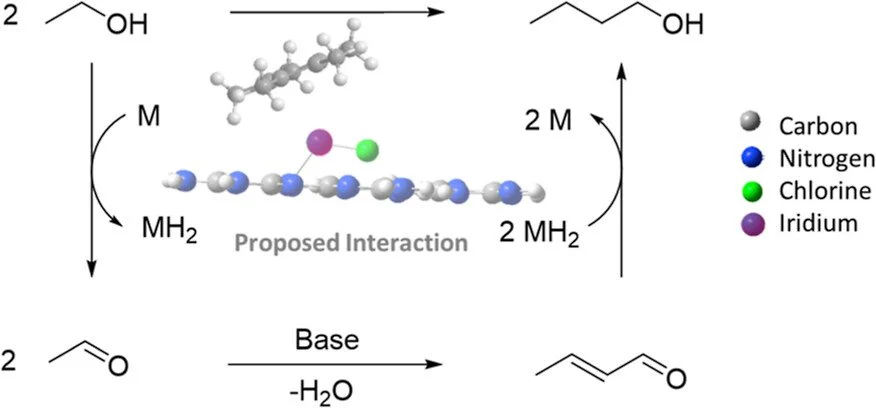
The upgrading of ethanol to n-butanol was performed using a molecular catalyst integrated into a carbon nitride support, one of the first examples of a supported molecular catalyst performing the Guerbet process. Initial studies using crystalline poly(triazine)imide (PTI) with lithium or transition-metal cations imbedded in the support together with a base as the catalyst system did not produce any significant amounts of n-butanol. However, when using the catalyst material formed by treatment of PTI-LiCl with [(Cp*)IrCl₂]₂ (Cp* = pentamethylcyclopentadienyl) along with sodium hydroxide, a 59% selectivity for butanol (13% yield) was obtained at 145 °C. This PTI-(Cp*)Ir material exhibited distinct UV–vis absorption features and powder X-ray diffractions which differ from those of the parent PTI-LiCl and [(Cp*)IrCl₂]₂. The PTI-(Cp*)Ir material was found to have a metal loading of 27% iridium per empirical unit of the framework. Along with the formation of n-butanol from the Guerbet reaction, the presence of higher chain alcohols was also observed.
Cypher, S. M.; Pauly, M.; Castro, L. G.; Donley, C. L.; Maggard, P. A.; Goldberg, K. I. Ethanol Upgrading to n-Butanol Using Transition-Metal-Incorporated Poly(triazine)imide Frameworks. ACS Appl. Mater. Interfaces 2023, 15 (30) 36384–36393. https://doi.org/10.1021/acsami.3c07396