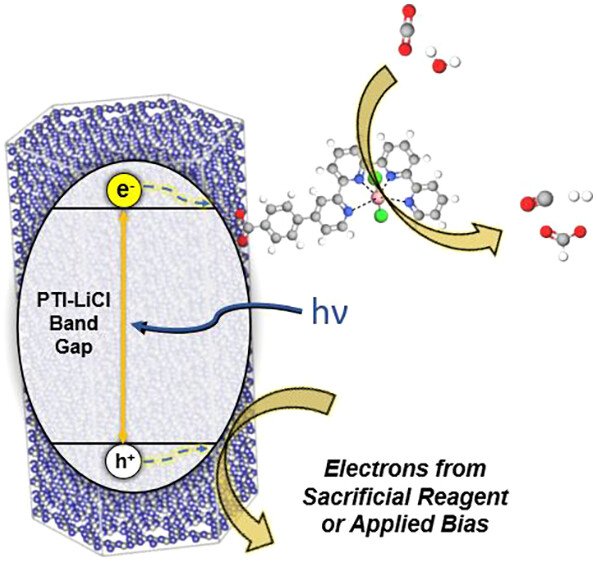
Discovery of a Hybrid System for Photocatalytic CO₂ Reduction via Attachment of a Molecular Cobalt-Quaterpyridine Complex to a Crystalline Carbon Nitride
While recent reports have demonstrated the attachment of molecular catalysts to amorphous, graphitic carbon nitrides (g-CN) for light-driven CO₂ reduction, approaches to the utilization of crystalline carbon nitrides have remained undiscovered. Herein, a functional hybrid photocatalyst system has been found using a crystalline carbon nitride semiconductor, poly(triazine imide) lithium chloride (PTI-LiCl), with a surface-attached CoCl₂(qpy-Ph-COOH) catalyst for CO₂ reduction. The molecular catalyst attaches to PTI-LiCl at concentrations from 0.10 to 4.30 wt % and exhibits ∼96% selectivity for CO production in a CO₂-saturated, aqueous 0.5 M KHCO3 solution. Optimal loadings were found to be within 0.42–1.04 wt % with rates between 1,400 and 1,550 μmol CO/g·h at an irradiance of 172 mW/cm² (λ = 390 nm) and apparent quantum yields of ∼2%. This optimized loading is postulated to represent a balance between maximal turnover frequency (TOF; 300+ h⁻¹) and excess catalyst that can limit excited-electron lifetimes, as probed via transient absorption spectroscopy. An increase in the incident irradiance yields a concomitant increase in the TOFs and CO rates only for the higher catalyst loadings, reaching up to 2,149 μmol CO/g·h with a more efficient use of the catalyst surface capacity. The lower catalyst loadings, by comparison, already function at maximal TOFs. Higher surface loadings are also found to help mitigate deactivation of the molecular catalysts during extended catalytic testing (>24 h) owing to the greater net surface capacity for CO₂ reduction, thus representing an effective strategy to extend lifetime. The hybrid particles can be deposited onto an FTO substrate to yield ∼60% Faradaic efficiency for photoelectrochemical CO production at −1.2 V vs Ag/AgCl bias. In summary, these results demonstrate the synergistic combination of a crystalline carbon nitride with a molecular catalyst that achieves among the highest known rates in carbon-nitride systems for the light-driven CO₂ reduction to CO in aqueous solution with >95% selectivity.
McGuigan, S.; Tereniak, S.; Donley, C.; Smith, A.; Jeon, S.; Zhao, F.; Sampaio, R.; Pauly, M.; Keller, L.; Collins, L.; Parsons, G.; Lian, T.; Stach, E.; Maggard, P. A. Discovery of a Hybrid System for Photocatalytic CO2 Reduction via Attachment of a Molecular Cobalt-Quaterpyridine Complex to a Crystalline Carbon Nitride. ACS Appl. Energy Materials. 2023, 6 (20), 10542-10553. https://doi.org/10.1021/acsaem.3c01670.