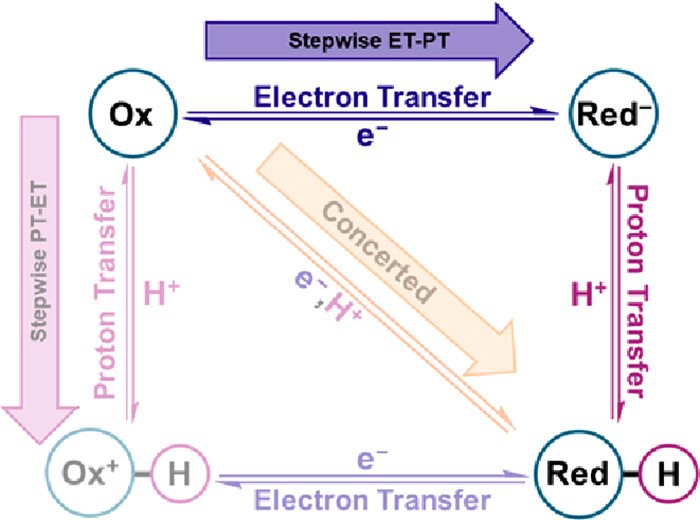
The proton-coupled electron transfer (PCET) mechanism for the reaction Mox–OH + e– + H+ → Mred–OH2 was determined through the kinetic resolution of the independent electron transfer (ET) and proton transfer (PT) steps. The reaction of interest was triggered by visible light excitation of [RuII(tpy)(bpy′)H2O]2+, RuII–OH2, where tpy is 2,2′:6′,2″-terpyridine and bpy′ is 4,4′-diaminopropylsilatrane-2,2′-bipyridine, anchored to In2O3:Sn (ITO) thin films in aqueous solutions. Interfacial kinetics for the PCET reduction reaction were quantified by nanosecond transient absorption spectroscopy as a function of solution pH and applied potential. Data acquired at pH = 5–10 revealed a stepwise electron transfer–proton transfer (ET–PT) mechanism, while kinetic measurements made below pKa(RuIII–OH/OH2) = 1.3 were used to study the analogous interfacial reaction, where electron transfer was the only mechanistic step. Analysis of this data with a recently reported multichannel kinetic model was used to construct a PCET zone diagram and supported the assignment of an ET–PT mechanism at pH = 5–10. Ultimately, this study represents a unique example among Mox–OH/Mred–OH2 reactivity where the protonation and oxidation states of the intermediate were kinetically and spectrally resolved to firmly establish the PCET mechanism.